Sodium Hypochlorite Generator works on electrochlorination chemical process which uses water, common salt and electricity to produce Sodium Hypochlorite(NaOCl). The brine solution (or sea water) is made to flow through an electrolyzer cell, where direct current is passed which leads to Electrolysis. This produces Sodium Hypochlorite instantaneously which is a strong disinfectant. This is then dosed in water in the required concentration to disinfect water, or to prevent Algae Formation and Bio Fouling. Pristine Water is the leading manufacturer of On-site Sodium Hypochlorite Generator.
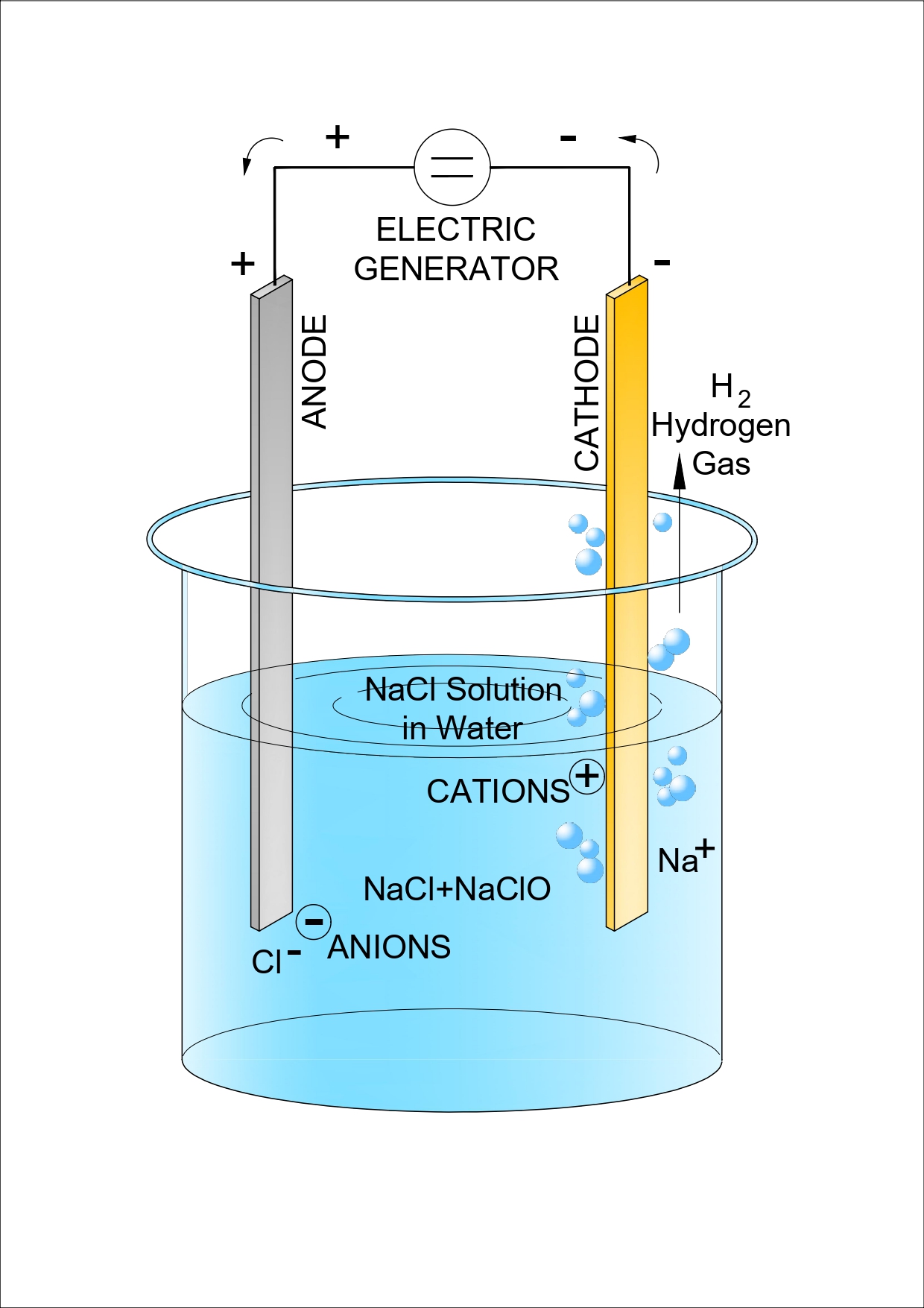
In the Electrolyser, the current is passed through the anode and cathode in the salt solution. which is a good conductor of electricity, thus electrolyzing the sodium chloride solution.
This results in chlorine (Cl2) gas being produced at the anode, while sodium hydroxide (NaOH) and hydrogen (H2) gas is produced at the cathode.
The reactions that take place in the electrolytic cell are:
2NaCl + 2H2O → 2NaOH + Cl2 + H2
The chlorine further reacts with the hydroxide to form sodium hypochlorite (NaOCl). This reaction can be simplified in the following manner:
Cl2 + 2NaOH → NaCl + NaClO + H2O
The solution generated has a pH value between 8 and 8.5, and a maximum equivalent chlorine concentration of less than 8 g/l. It has a very long shelf life which makes it suitable for storage.
After dosing the solution into the water flow, no pH value correction is necessary, as is often required in sodium hypochlorite produced by the membrane method. The sodium hypochlorite solution reacts in a balance reaction, resulting in hypochlorous acid:
NaClO + H2O → NaOH + HClO
To produce 1kg equivalent of chlorine using an on-site Sodium Hypochlorite generator, 4.5 kg of salt and 4-kilowatt hours of electricity is required. The final solution consists of approximately 0.8% (8 grams/liter) sodium hypochlorite.
Sodium Hypochlorite generated on-site with the help of synthetic brine or seawater is very efficient in protecting the equipment from the growth of micro-organic fouling and control of algae and crustaceans. Compact Electrochlorinators manufactured by Pristine Water are ideal for the disinfection of water during disasters like earthquakes, Floods, or Epidemics. Electrochlorinators are designed for rural and village “point-of-use” disinfection of drinking water. |
Although the economic consideration is the major advantage in using On-site generated Sodium Hypochlorite over the use of other forms of Chlorination, the technical advantages are even greater.
The following are some of the problems associated with using commercial-grade liquid sodium hypochlorite. These have a high concentration (10-12%) of active chlorine. These are produced by bubbling gas chlorine in Caustic soda (Sodium Hydroxide). They are also commonly called Liquid Chlorine.
The corrosion due to Commercially produced hypochlorite is a concern because of its effect on the equipment. A 10 to 15% hypochlorite solution is very aggressive due to its high pH and chlorine concentration. Because of its aggressive nature, the hypochlorite solution will exploit any weakened areas in the hypochlorite piping system and may cause leaks. So using an On-site sodium Hypochlorite generator is a wise option.
The formation of calcium carbonate scale is another concern when using commercial grade liquid hypochlorite for chlorination. Commercial grade liquid hypochlorite has a high pH. When the high pH hypochlorite solution is mixed with the dilution water, it raises the pH of the mixed water to above 9. The calcium in the water will react and precipitate out as calcium carbonate scale. Items such as pipes, valves, and rotameters may scale up and no longer function properly. It is recommended that the commercial-grade liquid hypochlorite not be diluted and that the smallest pipelines, the flow rate will allow, should be used in the system.
Another concern with commercial-grade hypochlorite is gas production. Hypochlorite loses strength over time and generates oxygen gas as it decomposes. The rate of decomposition increases with concentration, temperature, and metal catalysts.
A small leakage in the hypochlorite feed lines would result in the evaporation of the water and in turn the release of chlorine gas.
The final area of concern is the possibility of chlorate ion formation. Sodium hypochlorite degrades over time to form the chlorate ion (ClO3-) and oxygen (O2). The degradation of the hypochlorite solution is dependent on the strength of the solution, temperature, and the presence of metal catalysts.
Decomposition of Commercial Sodium Hypochlorite can be created in two major ways: a). The formation of Chlorates due to high pH, 3NaOCl= 2NaOCl+NaClO3. b). Chlorine evaporation loss due to temperature increase.
Therefore, for any given strength and temperature, over a period of time, the higher strength product will eventually be lower in available chlorine strength than the lower strength product, since its decomposition rate is greater. The American Water Works Association Research Foundation (AWWARF) concluded that the decomposition of concentrated bleach (NaOCl) is the most probable source of chlorate production. A high concentration of Chlorate is not advisable in drinking water.
| Product Form | pH Stability | Available Chlorine | Form |
| Cl2 gas | Low | 100% | Gas |
| Sodium hypochlorite (Commercial) | 13+ | 5-10% | Liquid |
| Calcium hypochlorite granular | 11.5 | 20% | Dry |
| Sodium hypochlorite (On-site) | 8.7-9 | 0.8-1% | Liquid |
Pristine Water offers sodium hypochlorite generator systems that are very effective, budget-friendly, safe, easy to prepare and use.
Our design team will be delighted to create a customized solution for you. Contact us here.